Atoms are so small that it's difficult to understand them. This interactive animation (click here) helps you to visualise their size. When it loads. Scroll to the left to see their size.
Lesson summary:
Parts of the atom
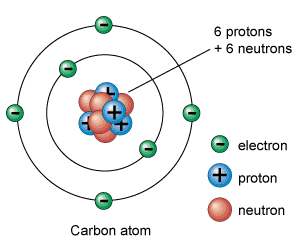
This is the Bohr model of the atom. At GCSE, this is a good model to use.
Note that protons and neutrons are in the nucleus. Electrons orbit the nucleus and are in shells or orbitals (or energy levels).
Protons have a positive charge. Neutrons have no charge. Electrons have no charge and are attracted to protons; opposites attract and like charges repel.
| Sub-atomic particle | Relative Mass | Charge | Location |
|---|---|---|---|
| Proton | 1 | +1 | In the nucleus |
| Neutron | 1 | 0 | In the nucleus |
| Electron | 1/2000 | -1 | Orbiting the nucleus |
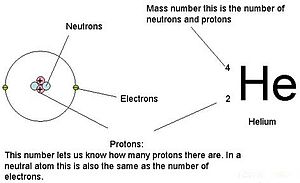
Adding the number of protons and neutrons together gives you the atomic mass (or mass number) of an element.
Elements also have an atomic number which tell you the number of protons in an atom.
This diagram helps:
Elements also have an atomic number which tell you the number of protons in an atom.
This diagram helps:
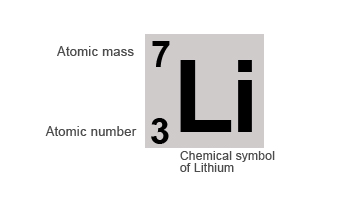
You can calculated the number of neutrons in an atom given the mass number and the atomic number and taking the difference between them.
E.g. Lithium has atomic mass 7 and atomic number 3. Take the difference between these to get the number of neutrons, in this case, 4.
This video is a nice summary of the structure of the atom. Click here.
E.g. Lithium has atomic mass 7 and atomic number 3. Take the difference between these to get the number of neutrons, in this case, 4.
This video is a nice summary of the structure of the atom. Click here.
No comments:
Post a Comment